HARD
Earn 100
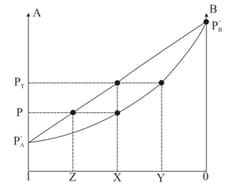
Mole fraction of liquid in liquid solution.
Mole fraction of vapour in vapour mixture.
is the graphical mid point between .
Find value of . [Given ]
If of
of
Give your answer after multiplying with 10 and round off to the nearest integer.

50% studentsanswered this correctly
Important Questions on Solutions
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
(molar mass of urea )
EASY
EASY
HARD
HARD
EASY
HARD
EASY
EASY
EASY
HARD
HARD
(Given that the vapour pressure of pure liquid A is at temperature )
HARD
MEDIUM
MEDIUM
EASY

