methyl--cyclohexanedione is more acidic than cyclohexanone - explain with reason
Explain why will add to the double bond in but not in

Important Questions on Aldehydes, Ketones and Carboxylic Acids
Convert
into
Identify and in the following reaction.
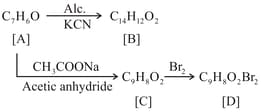
Complete the following reactions:
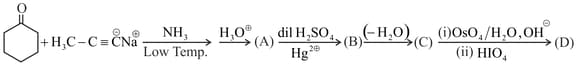
Write the structures of to and give the name of
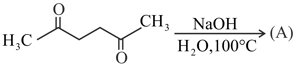
Give reasons for the following :
Ketones are less electrophilic than aldehydes.
Aldehydes are reducing agents and ketones are not.
fails to give addition products with carbonyl compounds.
Identify and in the following sequence of reactions:
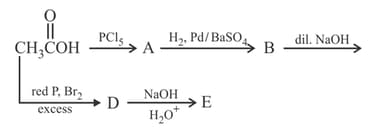
When the compound shown was heated in refluxing hydrochloric acid, a compound with the molecular formula was isolated. Identify this product. Along with this product, three other carbon-containing substances are formed. What are they ?
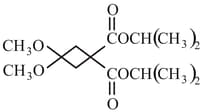
What happens when reacts with
follwed by hydrolysis
soda
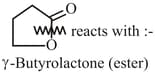
What are the product in each case?
