EASY
NEET
IMPORTANT
Earn 100
(a) Prove that for a monatomic gas the ratio of molar specific heat at constant pressure to molar specific heat at constant volume is
(b) Is it possible to boil water without heating? Explain.

Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
If denotes the specific heats of nitrogen per unit-mass at constant pressure and constant volume respectively, then :
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Two samples of and are initially kept in the same state. The sample is expanded through an adiabatic process and the sample through an isothermal process up to the same final volume. The final pressures in and are and respectively. Then,
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
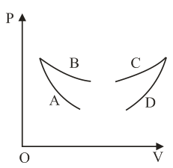
Four curves and are drawn in the figure for a given amount of gas. The curves which represent adiabatic and isothermal changes are