>This question is about the following oxides. Aluminium oxide , Calcium oxide , Carbon dioxide, Carbon monoxide , `Magnesium oxide , Sulphur dioxide
(iv) Which will react neither with hydrochloric acid nor with aqueous sodium hydroxide?

Important Questions on Cambridge IGCSE Exam Questions from Paper 3
| bond | bond energy in |
Use the above data to show that the following reaction is exothermic.
Hydrogen reacts with the halogens to form hydrogen halides. They react with water to form acidic solutions.
Explain why water behaves as a base in both of these reactions.
Hydrogen reacts with the halogens to form hydrogen halides. They react with water to form acidic solutions.
At equilibrium, only of the hydrogen chloride exists as molecules, the rest has formed ions. In the other equilibrium, of the hydrogen fluoride exists as molecules, only has formed ions. What does this tell you about the strength of each acid?
Hydrogen reacts with the halogens to form hydrogen halides. They react with water to form acidic solutions.
How would the pH of these two solutions differ?
The reactivity series lists metals in order of reactivity. To find out which is more reactive, zinc or tin, this experiment could be carried out.
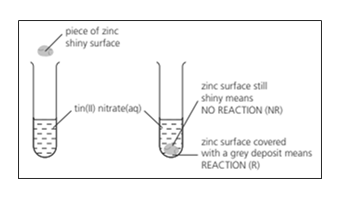
The experiment could be carried out with other metals and the results recorded in a table. Then the order of reactivity can be deduced. The order was found to be:
| aqueous solution | Tin | Manganese | Silver | Zinc |
| tin nitrate | - | |||
| manganese nitrate | ||||
| silver nitrate | ||||
| zinc nitrate |
Copy and complete this table of results from which the order was determined.
The reactivity series lists metals in order of reactivity. To find out which is more reactive, zinc or tin, this experiment could be carried out.
Write the ionic equation for the reaction between tin atoms and silver ions.
