MEDIUM
Earn 100
'a' and 'b' are Van der Waals' constants for gases. Chlorine is more easily liquefied than ethane because
(a)a and b for Cl2 > a and b for C2H6
(b)a and b for Cl2 < a and b for C2H6
(c)a for Cl2 < a for C2H6 but b for Cl2 > b for C2H6
(d)a for Cl2 > a for ethane but b for Cl2 < b for ethane
50% studentsanswered this correctly
Important Questions on States of Matter
HARD
MEDIUM
MEDIUM
HARD
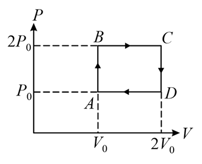
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
EASY
Given below are two statement : one is labelled as Assertion and the other is labelled as Reason .
Assertion is adsorbed to a large extent than on activated charcoal.
Reason : has a higher critical temperature than
In the light of the above statements, choose the most appropriate answer from the options given below.
HARD
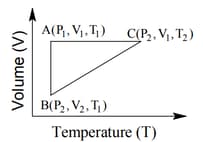
The correct option(s) is (are)
EASY
(Latent heat of ice is and )
EASY
MEDIUM
EASY
Among the following gases, the order of liquefiability is
a)
b)
c)
d)
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
EASY
MEDIUM
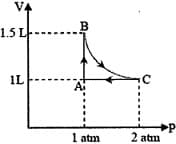
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is
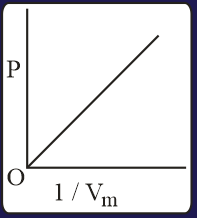
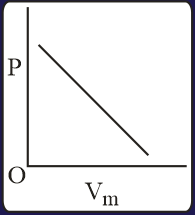
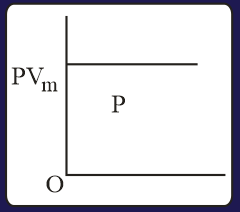
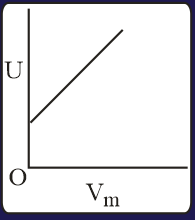
EASY
EASY
MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
EASY
Which of the following gases can be absorbed in more proportion?
MEDIUM
MEDIUM
| Gas | ||||
|---|---|---|---|---|
Which gas is expected to have the highest critical temperature?

