Answer the following, giving a full explanation in terms of metallic bonding:
Explain why aluminium has a higher melting point than sodium.

Important Questions on Chemical Bonding
Answer the following, giving a full explanation in terms of metallic bonding:
The thermal conductivity of stainless steel is 82 W m-1 K-1 . The thermal conductivity of copper is 400 W m-1 K-1. Why do some stainless steel saucepans have a copper base?
Answer the following, giving a full explanation in terms of metallic bonding:
Why does aluminium conduct electricity better than sodium?
Is the following molecule polar or non-polar? Give a reason for your answer. (Electronegativity values: F = 4.0, Cl = 3.0, Br= 2.8, S =2.5, C = 2.5, H=2.1)
chlorine, Cl2
Is the following molecule polar or non-polar? Give a reason for your answer. (Electronegativity values: F = 4.0, Cl = 3.0, Br= 2.8, S =2.5, C = 2.5, H=2.1)
hydrogen fluoride, HF
Is the following molecule polar or non-polar? Give a reason for your answer. (Electronegativity values: F = 4.0, Cl = 3.0, Br= 2.8, S =2.5, C = 2.5, H=2.1)
The V-shaped molecule, sulfur dichloride, SCI2
Is the following molecule polar or non-polar? Give a reason for your answer. (Electronegativity values: F = 4.0, Cl = 3.0, Br= 2.8, S =2.5, C = 2.5, H=2.1)
The tetrahedral molecule, chloromethane, CH3Cl
Is the following molecule polar or non-polar? Give a reason for your answer. (Electronegativity values: F = 4.0, Cl = 3.0, Br= 2.8, S =2.5, C = 2.5, H=2.1)
The tetrahedral molecule, tetrabromomethane, CBr4
The boiling points of the halogens are:
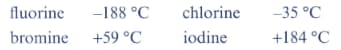
Describe the trend in these boiling points going down Group 17.
