HARD
Earn 100
Which of the following are macromolecular colloids?
(a)Starch
(b) Soap
(c) Detergent
(d) Cellulose
83.33% studentsanswered this correctly
Important Questions on Surface Chemistry
EASY
EASY
Match the following:
| (i) Foam | (a) smoke |
| (ii) Gel | (b) cell fluid |
| (iii) Aerosol | (c) jellies |
| (iv) Emulsion | (d) rubber |
| (e) froth | |
| (f) milk |
MEDIUM
Identify the correct molecular picture showing what happens at the critical micellar concentration of an aqueous solution of a surfactant

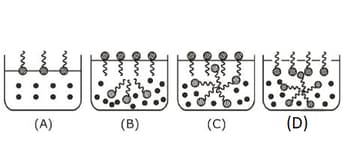
EASY
EASY
MEDIUM
EASY
Match List I with List II :
List-I List-II
Example of colloids Classification
(a) Cheese (i) dispersion of liquid in liquid
(b) Pumice stone (ii) dispersion of liquid in gas
(c) Hair cream (iii) dispersion of gas in solid
(d) Cloud (iv) dispersion of liquid in solid
Choose the most appropriate answer from the options given below
EASY
MEDIUM
(Critical micelle concentration (CMC) is marked with an arrow in the figures.)
EASY
EASY
EASY
EASY
Match the following:
| List- | List- | ||
| Type of Colloid | Phase in medium | ||
| () | Aerosol | () | Solid in solid |
| () | Emulsion | () | Liquid in solid |
| () | Foam | () | Gas in liquid |
| () | Gel | () | Solid in gas |
| () | Liquid in liquid |
The correct answer is
EASY
EASY
MEDIUM
EASY
MEDIUM
MEDIUM

