Which of the statements is/are true about the reactivity of halogenation of alkanes? The reactivity order is
I. Lower the activation energy for the chain initiation step, more reactive is the halogen.
II. Lower the activation energy for the first chain propagation step, more reactive is the halogen
III. More negative is the overall heat of the reaction of halogenation of alkane, more reactive is the halogen
IV. Lower the activation energy for the second chain propogation step, more reactive is the halogen.
Important Questions on Hydrocarbons
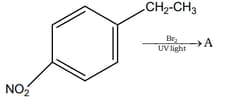
Which hydrogen in compound is easily replaceable during bromination reaction in presence of light ?
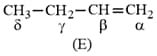
Presence of which reagent will affect the reversibility of the following reaction, and change it to a irreversible reaction :
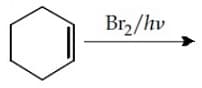
The total number of monohalogenated organic products in the following (including stereoisomers) reaction is
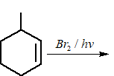
Given below are two statements:
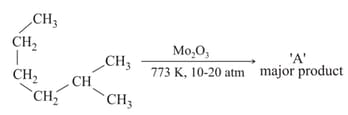
Statement-I : -methylbutane on oxidation with gives -methylbutan--ol.
Statement-II : -alkanes can be easily oxidised to corresponding alcohol with .
Choose the correct option :
Which of the following is a free radical substitution reaction?