moles of gas dissolves completely in water at , as shown in the following figure.
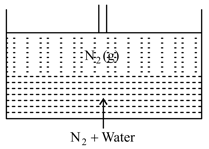
The available space between piston and liquid solution is . Find the number of moles of in gaseous phase.
for gas , Round off the value to the nearest integer.

for gas , Round off the value to the nearest integer.

Important Questions on Solutions
In an Ostwald-Walker experiment, dry air was first blown through a solution containing a certain amount of solute in of water and then also through pure water. The loss in mass of water was found to be , while the mass of water absorbed in sulphuric acid was . Calculate the amount of the solute.
Give your answer up to two decimal places without rounding-off.
To of water, of acetic acid is added. If of acetic acid is dissociated, what will be the depression in freezing point? and density of water are and respectively.
Give answer after multiplying with 100 and rounding off to the nearest integer value.
A solution of a nonvolatile solute in water freezes at . The vapour pressure of pure water at is and for water is . Calculate the magnitude of change in vapour pressure of this solution at .
Give answer after multiplying with 100 and rounding off to the nearest integer value.
A solution of an acid (density ) is ionised. Calculate the depression in fp of the solution. The molecular weight of the acid is .
Give answer after multiplying with 100 and rounding off to the nearest integer value.
An aqueous solution of mannitol in water has a vapour pressure of at , at which temperature the vapour pressure of pure water is . What is the f.p. depression for this solution?
Give answer after multiplying with 100 and rounding off to the nearest integer value.
The freezing point of a solution containing of acetic acid in of benzene is lowered by . Calculate the degree of association of acetic acid in benzene. .
[Hint: Acetic acid exists as dimer in benzene)The tallest trees known are the redwoods in California. Assuming the height of a redwood tree to be , estimate the osmotic pressure required to push water up from the roots to the treetop.
If the answer is of type , mention the value of correct up to nearest integer value.
A solution of crab hemocyanin, a pigmented protein extracted from crabs was prepared by dissolving in of an aqueous medium. At , the osmotic pressure rise of of the solution was observed. The solution had a density of . Determine the molecular weight (in g mole-1) of the protein.
Report your answer after dividing it by and rounding-off up to nearest integer.
