EASY
Earn 100
0.6 mole of mole of and 0.4 mole of are taken in a 1 L flask to obtain the following equilibrium
If the equilibrium constant for the reaction is 0.15. Predict the direction of the reaction.
(a)Forward direction
(b)Backward direction
(c)Direction of the reaction can not be predicted
(d)Reaction does not move in any direction
53.85% studentsanswered this correctly
Important Questions on Equilibrium
EASY
.....(1)
.....(2)
The relation between and is:
MEDIUM
moles each of hydrogen and iodine is heated in a sealed ten litre vessel.At equilibrium, moles of were found. The equilibrium constant for is ........
HARD
(At )
MEDIUM
The equilibrium constant () of the reaction:
, will be:
MEDIUM
EASY
MEDIUM
EASY
(assuming ideality)
EASY
The rate of this reaction is increased by
MEDIUM
When and are compared at It is found that
MEDIUM
MEDIUM
The value of for the reaction
The value of for the following reaction is :
MEDIUM
.... (i)
.... (ii)
are related as
EASY
MEDIUM
EASY
A solid kept in an evacuated sealed container undergoes decomposition to form a mixture of gases at temperature .
The equilibrium pressure is in this vessel. for this reaction is?
MEDIUM
EASY
For the reaction:
is
HARD
The reaction
is in equilibrium in a closed vessel at The partial pressure (in atm) of in the reaction vessel is closest to:
[Given: The change in Gibbs energies of formation at and bar for
Gas constant ]
EASY
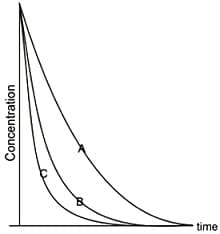
These profiles imply that the decay constants and follow the order

