of each of the elements with atomic numbers to was burned in chlorine gas. In each case there was an exothermic reaction and the amount of energy released was measured. The results are shown in the table.
Atomic number
Energy released
The element with atomic number did not react with chlorine gas. Suggest why is it so?
Important Questions on Chemical reactions and Equations
A metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle.
Write a balanced equation for the reaction if one of the compounds formed is calcium chloride.
Choose the method of preparation of Iron (II) sulphate, from the methods given in the list:
[List: A. Neutralization B.Precipitation C.Direct combination D.Substitution]
Which of the following are combination reaction?
(i)
(ii)
(iii)
(iv)
Choose the method of preparation of Iron (III) chloride, from the methods given in the list:
[List: A. Neutralization B.Precipitation C.Direct combination D.Substitution]
Consider the following equation of the chemical reaction of a metal M
The equation represents:-
Which of the following is a reversible reaction?
This reaction is
| List- (Chemical reactions) | List- (Type of chemical reactions) |
| Addition | |
| Elimination | |
| Redox | |
| Substitution |
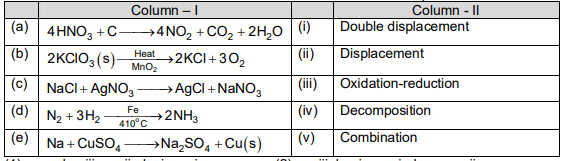
Match the items of the column -I with column-II and choose the correct option.
Calcium oxide reacts vigorously with water to produce slaked lime.
This reaction can be classified as:
(A) Combination reaction (B) Exothermic reaction
(C) Endothermic reaction (D) Oxidation reaction
Which of the following is the correct answer?

