HARD
Earn 100
of an ideal gas, , are taken through a series of processes.
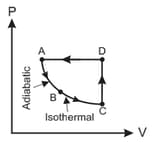
Information-I The temperature at state '' is .
Information-II The reversible isothermal expansion from doubles the volume.
Information-III The entropy change of the system from is .
Select the correct option(s) using the above information.
(a)The entropy change of the system from is .
(b)The temperature at point is .
(c)The work done from is .
(d)The work done from is .
11.11% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
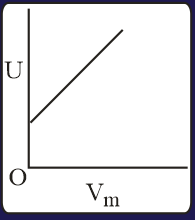
The combination of plots which does not represent isothermal expansion of an ideal gas is
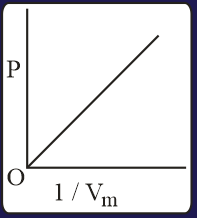
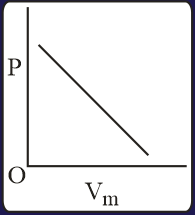
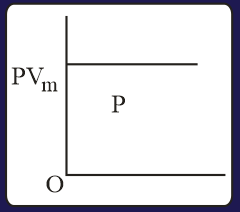
HARD
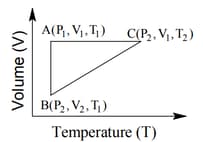
The correct option(s) is (are)
HARD
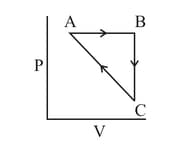
Heat absorbed by the system during process is
HARD
MEDIUM
MEDIUM
HARD
One mole of an ideal monoatomic gas is subjected to changes as shown in the graph. The magnitude of the work done (by the system or on the system) is _______ J (nearest integer)
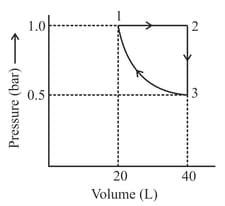
Given : log 2 = 0.3, ln 10 = 2.3
EASY
HARD
MEDIUM
Above shows a cyclic process. Calculate the total work done during one complete cycle.
(Assume a single step to reach the next state).
HARD
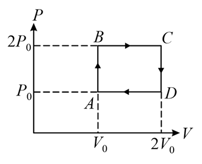
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
MEDIUM
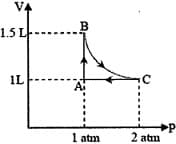
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
EASY
(Latent heat of ice is and )
EASY
EASY
MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
MEDIUM
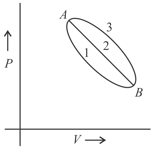
A certain mass of a gas was brought from state to by following three different paths, namely and , respectively. Which of the following relations is correct for the work done?
MEDIUM
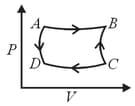
and are isothermal processes while and are adiabatic processes. The same cycle in the temperature - entropy plane is :
HARD
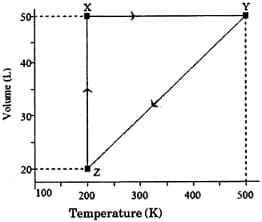
The pressure of the gas (in atm) at and respectively, are

