HARD
JEE Main
IMPORTANT
Earn 100
is added to of saturated solution of . The conductivity of this solution at is .
[Given : at
50% studentsanswered this correctly

Important Questions on Electrochemistry
EASY
JEE Main
IMPORTANT
The molar conductivity at infinite dilution of barium chloride, sulphuric arid and hydrochloric acid are respectively. The molar conductivity at infinite dilution of barium sulphate is ___ ( Round off to the Nearest Integer).
MEDIUM
JEE Main
IMPORTANT
For the reaction
the magnitude of the standard molar free energy change, ______ (Round off to the Nearest Integer).
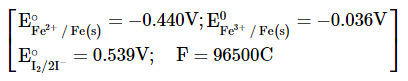
MEDIUM
JEE Main
IMPORTANT
A solution of conductivity shows a resistance of in a conductivity cell. If the same cell is filled with an solution, the resistance drops to . The conductivity of the HCl solution is ____. (Round off to the Nearest Integer).
HARD
JEE Main
IMPORTANT
A aqueous solution of has a conductance of when measured in a cell constant . The molar conductivity of this solution is _______ . (Round off to the Nearest Integer)
EASY
JEE Main
IMPORTANT
Amongst the following, the form of water with the lowest ionic conductance at is:
HARD
JEE Main
IMPORTANT
of silver (molar mass ) is deposited at cathode from solution by a certain quantity of electricity. The volume (in ) of oxygen gas produced at and bar pressure from water by the same quantity of electricity is . Value of to the nearest integer is _____.
MEDIUM
JEE Main
IMPORTANT
If the standard electrode potential for a cell is at the equilibrium constant for the reaction.
at is approximately:
MEDIUM
JEE Main
IMPORTANT
The anodic half-cell of lead-acid battery is recharged using electricity of Faraday. The amount of electrolyzed in during the process is: (Molar mass of )
