MEDIUM
Earn 100
is treated with two equivalents of in the presence of dry ether. Which of the following will be formed?
(a)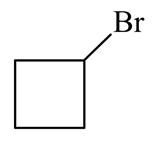

(b)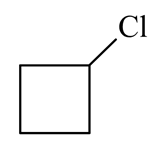

(c)

(d)

46.15% studentsanswered this correctly
Important Questions on Hydrocarbons
EASY
Wurtz reaction
Reduction of alkyl halides with zinc and dil.
Kolbe's electrolysis
Catalytic hydrogenation of alkenes
EASY
Which of the following reactions is used for producing symmetrical alkane with double carbon atoms?
MEDIUM
Assertion (A): Sodium acetate on Kolbe's electrolysis gives ethane.
Reason (R): Methyl free radical is formed at cathode.
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
State one relevant reason for the following:
- Soda-lime is preferred to sodium hydroxide in the laboratory preparation of methane.
HARD
Write a balanced chemical equation for the following:
- Producing ethane from bromo-ethane using couple in alcohol.
MEDIUM
MEDIUM

