HARD
KVPY Aptitude Test - Stream SA
IMPORTANT
Earn 100
mole of an ideal gas undergoes an isothermal expansion as energy is added to it as heat . Graph shows the volume versus . The gas temperature is nearly equal to (use mole)
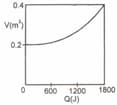

(a)
(b)
(c)
(d)
25% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases and Thermodynamics
HARD
KVPY Aptitude Test - Stream SA
IMPORTANT
Curve in the figure shows an adiabatic compression of an ideal gas from to , followed by an isothermal compression to a final volume of . There are moles of the gas. Total heat supplied to the gas is equal to :
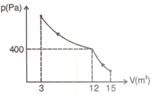
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The correct curve between and for an ideal gas at constant pressure is
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
A mixture of ideal gasses and are taken in the mass ratio of , respectively. Molar heat capacity of the mixture at constant pressure is
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
in a noncyclic process of an ideal gas. The process
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
An ideal monoatomic gas initially at undergoes an isobaric expansion at a pressure of . If the volume increases from to , then heat added to the gas and its final temperature respectively are
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
A non-conducting container is divided into two chambers that are separated by a valve. The left chamber contains one mole of a monatomic ideal gas. The right chamber is evacuated. At some instant, the valve is opened and the gas rushes freely into the right chamber. Which are of the following statements concerning this process is true?
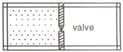
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
An ideal gas with adiabatic exponent undergoes a process in which work done by the gas is same as increase in internal energy of the gas. The molar heat capacity of gas for the process is
EASY
KVPY Aptitude Test - Stream SA
IMPORTANT
The ratio of specific heats at constant pressure and constant volume of a gas is . Then the average number of degree of freedom of the gas molecules is
