of Benzoic acid is dissolved in of Acetone and of Benzene separately. Boiling point of the solution in Acetone increases by while that of, in the Benzene increases by for Acetone and Benzene are and respectively. Molecular weight of Benzoic acid in Benzene is and in Acetone is . Calculate ?

Important Questions on Solutions
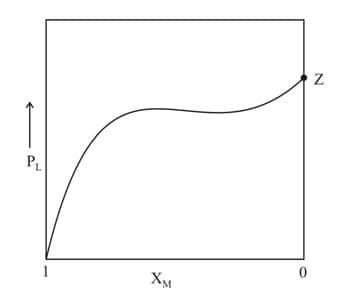
For a solution formed by mixing liquids and the vapour pressure of plotted against the mole fraction of in solution is shown in the following figure. Here and represent mole fractions of and respectively, in the solution. The correct statement(s) applicable to this system is (are):
The plot given below shows curves (where is the pressure and is the temperature) for two solvents and and isomolal solutions of in these solvents. completely dissociates in both the solvents.
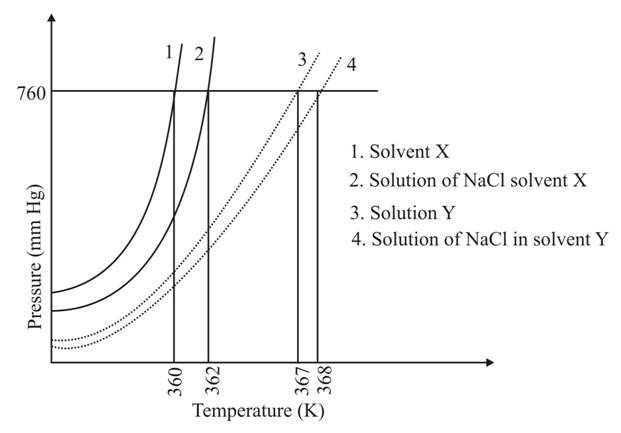
On addition of equal number of moles of a non-volatile solute in equal amount (in ) of these solvents, the elevation of boiling point of solvent is three times that of solvent Solute is known to undergo dimerization in these solvents. If the degree of dimerization is in solvent the degree of dimerization in solvent is:
