HARD
JEE Main/Advance
IMPORTANT
Earn 100
of a gaseous hydrocarbon was exploded with excess of . On cooling, the reaction mixture volume was reduced by , while on adding , the volume was reduced by . Molecular formula of hydrocarbon is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
HARD
JEE Main/Advance
IMPORTANT
A mixture of methane, propane and carbon monoxide contains propane by volume. If its are burnt in excess of , the volume of formed is :
HARD
JEE Main/Advance
IMPORTANT
A spherical balloon of diameter is to be filled with hydrogen at NTP from a cylinder containing the gas at and . If the cylinder can hold litres of water at NTP, calculate the number of balloons that can be filled up.
HARD
JEE Main/Advance
IMPORTANT
A column of Hg of 100 mm in length is contained in the middle of a narrow tube 1 m long which is closed at both ends. Both the halves of the tube contained air at a pressure of $760 \mathrm{mm}$ of Hg. By what distance (in $\mathrm{mm}$ ) will the column of Hg lie displaced if the tube is held vertical? Assume decrease in length of mercury column to be negligible, also take the process at constant temperature (isothermal process)
MEDIUM
JEE Main/Advance
IMPORTANT
Two vessels whose volumes are in the ratio contain nitrogen and oxygen at and pressures, respectively when they are connected together what will be the pressure of the resulting mixture (in meters)?
HARD
JEE Main/Advance
IMPORTANT
At two balloons of equal volume and porosity are filled to a pressure of one with and other with of balloon leaks to a pressure of in How long will it take for balloon to reach a pressure of ?
MEDIUM
JEE Main/Advance
IMPORTANT
A gas cylinder containing cooking gas can withstand a pressure of atmosphere. The pressure gauze of cylinder indicates atmospheres at . Due to sudden fire in the building, the temperature starts raising. The temperature at which cylinder will explode is
MEDIUM
JEE Main/Advance
IMPORTANT
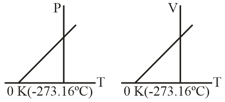
What conclusion would you draw from the following graphs for an ideal gas?
MEDIUM
JEE Main/Advance
IMPORTANT
An open-ended mercury manometer is used to measure the pressure exerted by a trapped gas as shown in the figure. Initially, manometer shows no difference in mercury level in both columns as shown in the diagram. After sparking dissociates according to the following reaction
If pressure of Gas decreases to
(Assume temperature to be constant and is )