HARD
JEE Main/Advance
IMPORTANT
Earn 100
of gaseous hydrocarbon is exploded with . The residual gas on cooling is found to measure of which is abosorbed by and the reminder by alkaline pyrogallol. The formula of the hydrocarbon is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
MEDIUM
JEE Main/Advance
IMPORTANT
The atom has times the mass of . Which of the following statements are true?
MEDIUM
JEE Main/Advance
IMPORTANT
Following represent the Maxwell distribution curve for an ideal gas at two temperature and Which of the following option(s) are true?
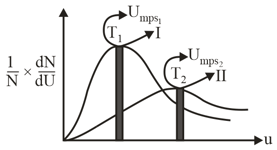
MEDIUM
JEE Main/Advance
IMPORTANT
If for two gases of molecular weights and at temperature and then which property has the same magnitude for both the gases?
MEDIUM
JEE Main/Advance
IMPORTANT
Gaseous decomposition of follows order kinetics. Pure is taken in a sealed flask where decomposition occurs as
After sec., a leak was developed in the flask. On analysis of the effused gaseous mixture (Obeying Graham's law) coming out initially, moles of were found to be double of . What is rate constant in
Given: Molecular weight of Molecular weight of Molecular weight of
EASY
JEE Main/Advance
IMPORTANT
Viscosity is a measure of resistance of a liquid to flow and viscosity-
MEDIUM
JEE Main/Advance
IMPORTANT
One mole of Ideal gas . follow the process as shown in figure. Predict the following:
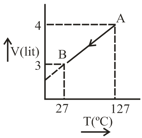
Nature of process
EASY
JEE Main/Advance
IMPORTANT
Internal pressure of a perfect gas (ideal gas) is:
