EASY
NEET
IMPORTANT
Earn 100
lit of water at was heated upto at constant pressure then calculate heat supplied. (If and and is not axis varies at this temperature internal).
(a)
(b)
(c)
(d)
66.67% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
plots for two gases during an adiabatic process are given in the figure:
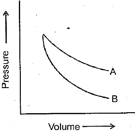
Plot and plot should correspond to: (Assume ideal behaviour).
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
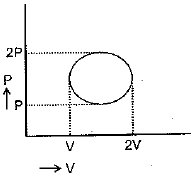
For this cyclic process change in enthalpy of ideal gas is:
MEDIUM
NEET
IMPORTANT
