of water is heated from to . Ignoring the slight expansion of the water, the change in its internal energy is (Specific heat of water is )

Important Questions on Thermodynamics
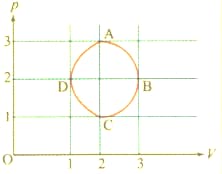
The figure shows the -plot of an ideal gas taken though a cycle . (Fig. ) The part is a semi-circle and ) is half of an ellipse. Then
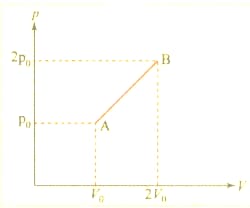
One mole of an ideal gas having initial volume , pressure and temperature under goes a cyclic process as shown in figure, The net work done in the complete cycle is
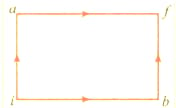
When a system is taken from state to state along the path , then found that and . Along the path , along the path is
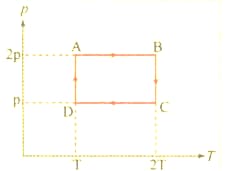
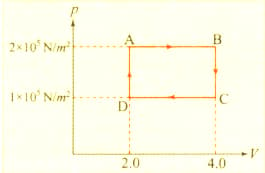
The , diagram of a gas undergoing a cyclic process , is shown in the graph, where , is in units of and in . Identify the incorrect statement.
Find the amount of heat required to raise the temperature of the gas through the same range at constant volume, of heat is required to raise the temperature of moles of an ideal gas at constant pressure from to .
The , diagram of , of helium gas for a certain process , is shown in the figure. What is the heat given to the gas during the process