EASY
JEE Main
IMPORTANT
Earn 100
of is taken in a beaker and to it of is added in steps of and the is continuously measured. Which of the following graphs correctly depicts the change in ?
(a)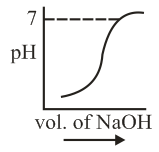

(b)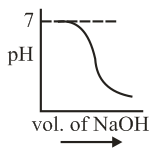

(c)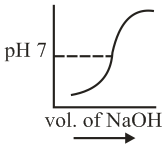
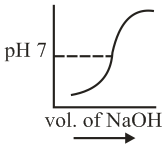
(d)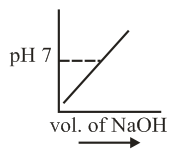

9.09% studentsanswered this correctly

Important Questions on Equilibrium
MEDIUM
JEE Main
IMPORTANT
An acidic buffer is obtained on mixing:
EASY
JEE Main
IMPORTANT
In the following reactions, is respectively acting as a/an,
(i)
(ii)
MEDIUM
JEE Main
IMPORTANT
of a weak acid and of a weak base are and respectively. The of their salt solution at is
EASY
JEE Main
IMPORTANT
The equilibrium constant for the reaction is At equilibrium, the partial pressure of is _____ atm. (Round off to the nearest integer)
EASY
JEE Main
IMPORTANT
moles of is introduced in a closed reaction vessel at The number of moles of at equilibrium is
(Round off to the Nearest Integer)
MEDIUM
JEE Main
IMPORTANT
When of solid is introduced into a two litre evacuated flask at of the solid decomposes into gaseous ammonia and hydrogen sulphide. The for the reaction at is . The value of is (Integer answer)
[Given
MEDIUM
JEE Main
IMPORTANT
The number of moles of that must be added to of in order to reduce the concentration of ions to for is __________. (Nearest integer)
[Assume no volume change on adding ]
MEDIUM
JEE Main
IMPORTANT
The of ammonium phosphate solution, if of phosphoric acid and of ammonium hydroxide are and respectively, is
