MEDIUM
JEE Main
IMPORTANT
Earn 100
of an organic compound which contains only carbon and hydrogen on complete combustion gives of and of water. The percentage of carbon and hydrogen in the organic compound are respectively
(a) and
(b) and
(c) and
(d) and
56.25% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
JEE Main
IMPORTANT
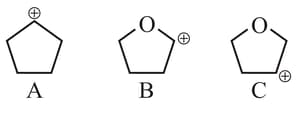
Arrange the following carbocations in decreasing order of stability.
HARD
JEE Main
IMPORTANT
of an organic compound was subjected to estimation of nitrogen by Dumas method in which volume of evolved (at STP) was found to be . The percentage of nitrogen in the compound is - [nearest integer] (Given: Molar mass of is , Molar volume of at )
MEDIUM
JEE Main
IMPORTANT
In the following structures, which one is having staggered conformation with maximum dihedral angle?
MEDIUM
JEE Main
IMPORTANT
During halogen test, sodium fusion extract is boiled with concentrated to
MEDIUM
JEE Main
IMPORTANT
The correct order of nucleophilicity is
MEDIUM
JEE Main
IMPORTANT
Given below are two statements :
Statement I : In 'Lassaigne's Test', when both nitrogen and sulphur are present in an organic compound, sodium thiocyanate is formed.
Statement II : If both nitrogen and sulphur are present in an organic compound, then the excess of sodium used in sodium fusion will decompose the sodium thiocyanate formed to give and .
In the light of the above statements, choose the most appropriate answer from the options given below
MEDIUM
JEE Main
IMPORTANT
Consider the above reaction and identify the intermediated ''
MEDIUM
JEE Main
IMPORTANT
Which will have the highest enol content?
