MEDIUM
NEET
IMPORTANT
Earn 100
oxygen gas expands at STP isobarically to occupy double of its original volume. The work done during the process is nearly
(a)
(b)
(c)
(d)
77.78% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Different types of systems are given below
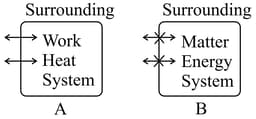
The and systems respectively are
