of ice at is mixed with of water at in an insulating vessel having a negligible heat capacity. Calculate the final mass of water remaining in the container. It is given that the specific heats of water and ice are and , while the latent heat of fusion of ice is .

Important Questions on Calorimetry and Heat Transfer
In an industrial process of water per hour is to be heated from to . To do this, steam at is passed from a boiler into a copper coil immersed in water. The steam condenses in the coil and is returned to the boiler as water at , how many of steam is required per hour.
(Specific heat of steam, , Latent heat of vaporisation, and specific heat of water, )
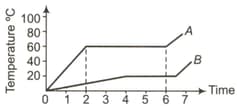
Which of the substances and has the lowest heat capacity, if heat is supplied to all of them at equal rates? The temperature versus time graph is shown below:
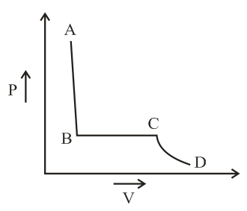
The portion of the indicator diagram representing the state of matter denotes
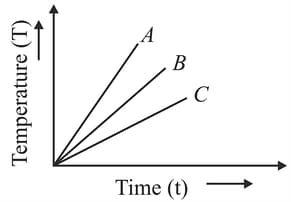
Two substances and of equal mass are heated at a uniform rate of under similar conditions. A graph between temperature and time is shown in the figure. The ratio of heat absorbed by them for complete fusion is