MEDIUM
9th Foundation
IMPORTANT
Earn 100
of molten sodium bromide is decomposed electrolytically. The maximum mass of sodium obtained is [Atomic mass of ]
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atoms and Molecules
MEDIUM
9th Foundation
IMPORTANT
I of carbon was burnt in of oxygen to give of .
II of carbon was burnt in air to give of .
III of carbon was burnt in enough air to give of .
IV of carbon was burnt in oxygen to form of .
Law of constant proportions is illustrated in experiment(s)
HARD
9th Foundation
IMPORTANT
Match column I with column II and mark the correct option from the given codes.
| Column I | Column II | ||
| (a) | of | (i) | |
| (b) | mole of | (ii) | |
| (c) | Molecular mass of common salt | (iii) | |
| (d) | mole of | (iv) |
EASY
9th Foundation
IMPORTANT
HARD
9th Foundation
IMPORTANT
HARD
9th Foundation
IMPORTANT
Which of the following represents the correct number of moles of each element in of ferric sulphate? [Atomic mass of ]
| (A) | |||
| (B) | |||
| (C) | |||
| (D) |
HARD
9th Foundation
IMPORTANT
HARD
9th Foundation
IMPORTANT
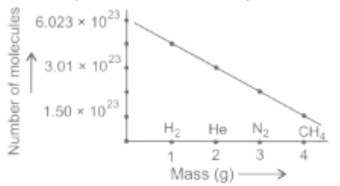
The graphical representation of number of molecules of different gases is given. Which gases are placed at correct position?
HARD
9th Foundation
IMPORTANT
