MEDIUM
JEE Main
IMPORTANT
Earn 100
of a waste solution obtained from the workshop of a goldsmith contains and . The solution was electrolyzed at by passing a current of for minutes. The metal/metals electrodeposited will be :
(a)only gold
(b)silver and gold in proportion to their atomic weights
(c)only silver
(d)silver and gold in equal mass proportion
100% studentsanswered this correctly

Important Questions on Electrochemistry
EASY
JEE Main
IMPORTANT
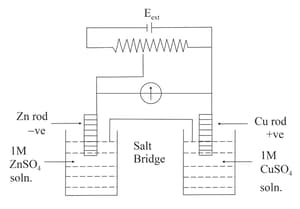
Identify the incorrect statement from the options below for the above cell:
EASY
JEE Main
IMPORTANT
Galvanization is applying a coating of:
MEDIUM
JEE Main
IMPORTANT
Match List - I with List - II :
| List - I (Parameter) |
List - II (Unit) | ||
| (a) | Cell constant | (i) | |
| (b) | Molar conductivity | (ii) | Dimensionless |
| (c) | Conductivity | (iii) | |
| (d) | Degree of dissociation of electrolyte | (iv) |
MEDIUM
JEE Main
IMPORTANT
Consider the following cell reaction
The value of is at If the standard entropy change in is _________ . (Nearest integer)
[Given : Faraday constant ]
MEDIUM
JEE Main
IMPORTANT
An acidic solution of dichromate is electrolysed for minutes using A current. As per the following equation
The amount of obtained was . The efficiency of the process is (Take : , At. mass of chromium )
MEDIUM
JEE Main
IMPORTANT
Let and , be the conductances (in S) measured for saturated aqueous solutions of and respectively, at a temperature T. Which of the following is false?
HARD
JEE Main
IMPORTANT
The photoelectric current from Na (work function, ) is stopped by the output voltage of the cell
the of aq. required to stop the photoelectric current from , all other conditions remaining the same, is (to the nearest integer).
Given
EASY
JEE Main
IMPORTANT
Given
Among the following, the strongest reducing agent is:
