HARD
10th ICSE
IMPORTANT
Earn 100
g of ferrous sulphate crystals were heated in a hard glass test -tube and observations were recorded.
Name the products obtained on heating ferrous sulphate crystals.
Important Questions on Chemical Reactions and Equations
HARD
10th ICSE
IMPORTANT
MEDIUM
10th ICSE
IMPORTANT
MEDIUM
10th ICSE
IMPORTANT
MEDIUM
10th ICSE
IMPORTANT
HARD
10th ICSE
IMPORTANT
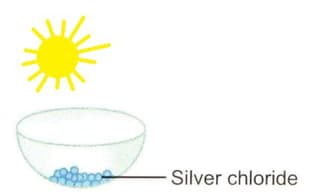
Identify the type of chemical reaction that will take place and define it. How will the colour of the salt change?
MEDIUM
10th ICSE
IMPORTANT
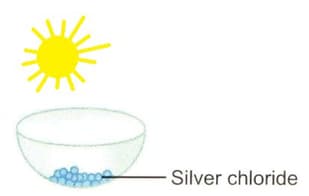
Write the chemical equation of the reaction that take place.
MEDIUM
10th ICSE
IMPORTANT
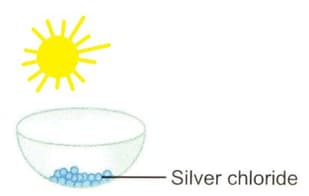
Mention one commercial use of this salt.
MEDIUM
10th ICSE
IMPORTANT
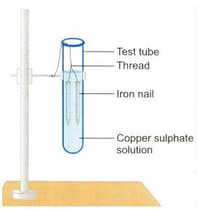
After ten minutes of keeping the set-up as shown in the figure, the colour of the iron nail changes, what does this indicate?
