EASY
NEET
IMPORTANT
Earn 100
moles of an ideal gas are contained within a cylinder by a frictionless piston and are initially at temperature The pressure of the gas remains constant while it is heated and its volume doubles. If is molar gas constant, the work done by the gas in increasing its volume is
(a) in
(b) in
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
The variation of pressure with volume for an ideal monatomic gas during an adiabatic process is shown in figure. At point the magnitude of rate of change of pressure with volume is
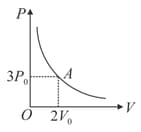
EASY
NEET
IMPORTANT
Figure shows, the adiabatic curve on a and scale performed on ideal gas. The gas is
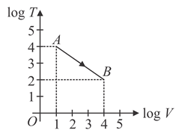
EASY
NEET
IMPORTANT
