of oxygen gas at temperature is compressed adiabatically to of its initial volume. Calculate the change in internal energy ( for oxygen).

Important Questions on Thermodynamics
An ideal gas expands isothermally along and does of work.
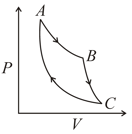
(a) How much heat does the gas exchange along .
(b) The gas expands adiabatically along and does of work. When the gas returns to , it exhausts of heat to its surroundings. How much work is done on the gas along this path.
For one mole of an ideal gas at occupies a volume of at a pressure of . The gas expands adiabatically until its volume becomes . Next, the gas is compressed isobarically to its original volume. Finally, the pressure is increased isochorically until the gas returns to as initial state (see figure).
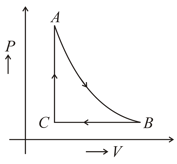
(a) Determine the temperature at the point .
(b) The work done during the process .
(Assume, .)
The following figure is given for gas.
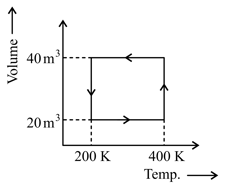
Then, find out net work done in the cyclic process.
