HARD
JEE Main
IMPORTANT
Earn 100
of hydrogen is ignited with of oxygen, the amount of water formed is?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Some Basic Concepts of Chemistry
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
Given that the abundance of isotopes of iron, and are , and, respectively, then calculate the atomic mass of .
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
The number of molecules of the sweetener saccharin, which can be prepared
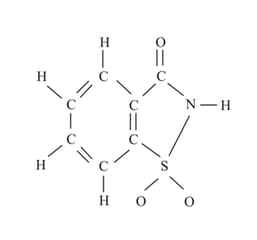
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
