MEDIUM
JEE Main
IMPORTANT
Earn 100
was dissolved in deionized water to prepare a stock solution. What volume (in mL) of this solution would be required to prepare of solution ? Given : Molar Mass of respectively
84.62% studentsanswered this correctly

Important Questions on Some Basic Concepts of Chemistry
MEDIUM
JEE Main
IMPORTANT
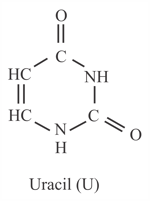
Uracil is base present in with the following structure. in uracil is _______.
MEDIUM
JEE Main
IMPORTANT
The number of units, which are used to express concentration of solutions from the following is _________.
(Mass percent, Mole, Mole fraction, Molarity, ppm, Molality.)
MEDIUM
JEE Main
IMPORTANT
The density of a monobasic strong acid (Molar mass is . The volume of its solution required for the complete neutralization of of is (Nearest integer)
EASY
JEE Main
IMPORTANT
In sulphur estimation. of an organic compound gave of barium sulphate. The percentage of sulphur in the compound is _______(Nearest Integer)
(Given: Atomic mass Ba: )
MEDIUM
JEE Main
IMPORTANT
What is the mass ratio of ethylene glycol (, molar mass ) required for making of molal aqueous solution and of molar aqueous solution?
MEDIUM
JEE Main
IMPORTANT
Number of hydrogen atoms per molecule of a hydrocarbon A having carbon is (Given : Molar mass of )
EASY
JEE Main
IMPORTANT
When a hydrocarbon undergoes combustion in the presence of air, it requires equivalents of oxygen and produces equivalents of water. What is the molecular formula of ?
MEDIUM
JEE Main
IMPORTANT
The volume of , containing , required to completely neutralise obtained by reacting of metallic sodium with water, is _____ . (Nearest Integer)
(Given : molar Masses of are and respectively)
