of zinc is treated separately with an excess of
(a). Dilute hydrochloric acid and
(b). Aqueous sodium hydroxide.
The ratio of the volumes of evolved in these two reactions is:
Important Questions on Metals and Non-Metals
Write a chemical equation for the reaction taking place in the flask. Write name and one property of the gas evolved.
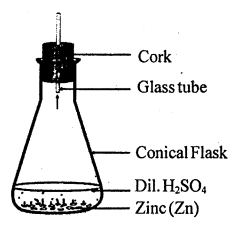
State one observation for the following:
A small piece of zinc is added to dilute hydrochloric acid.
Which gas is produced during the reaction in the test tube? How does this gas react with calcium hydroxide/lime water?
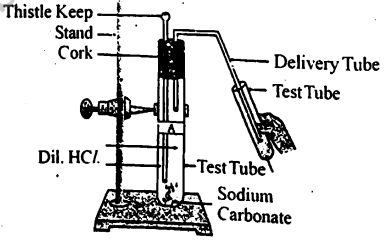
State one relevant observation for of the following:
Lead nitrate solution is mixed with dilute hydrochloric acid and heated.
Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
