EASY
Earn 100
50 mL of a gas A diffuses through a membrane in the same time as for the diffusion of 40 mL of a gas B under identical pressure and temperature conditions. If the molecular weight of A = 64, that of B would be
50% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
MEDIUM
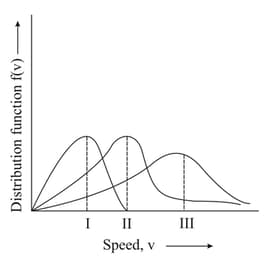
EASY
MEDIUM
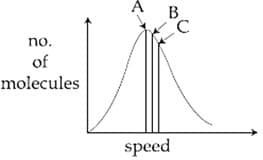
Root mean square speed most proable speed Average speed
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
