of heat is added to a monoatomic gas in a process in which the gas performs a work of . The molar heat capacity for the process is
Important Questions on Laws of Thermodynamics
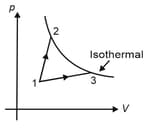
diagram of one mole of an ideal monoatomic gas is shown. Processes and are adiabatic. Work done in the complete cycle is
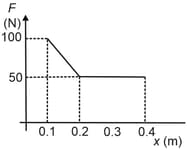
The given figure shows the variation of force applied by ideal gas on a piston which undergoes a process during which piston position changes from to . If the internal energy of the system at the end of the process is higher, then the heat absorbed during the process is
A thermodynamical process is shown in the figure with , , ,
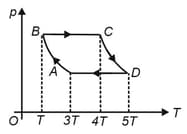
In the process and , and heat are added to the system. Find the change in internal energy of the system in the process .
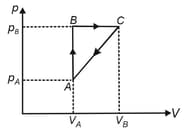
A gas takes part in two processes, in which it is heated from the same initial state to the same final temperature. The processes are shown on the diagram by the straight lines , and are the points on the same isothermal curve. and are the heat transfer along with the two processes. Then,