mole of certain monoatomic ideal gas undergoes a temperature increase of at constant pressure. The increase in the internal energy of the gas in this process is
(Given )

Important Questions on Thermodynamics
Read the following statements :
. When small temperature difference between a liquid and its surrounding is doubled the rate of loss of heat of the liquid becomes twice.
. Two bodies and having equal surface areas are maintained at temperature and . The thermal radiation emitted in a given time by and are in the ratio
. A carnot Engine working between and has an efficiency of
. When small temperature difference between a liquid and its surrounding is quadrupled, the rate of loss of heat of the liquid becomes twice.
Choose the correct answer from the options given below :
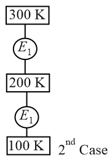
In case, Carnot engine operates between temperatures and . In case, as shown in the figure, a combination of two engines is used. The efficiency of this combination (in case) will be :
The pressure and density of diatomic gas changes suddenly to and respectively during an adiabatic process. The temperature of the gas increases and becomes____times of its initial temperature.
(Given )
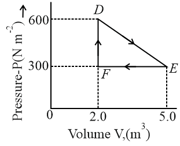
A thermodynamic system is taken from an original state to an intermediate state by the linear process shown in the figure. Its volume is then reduced to the original volume from to by an isobaric process. The total work done by the gas from to to will be