MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
mixture of , and heated strongly in an open vessel. After complete decomposition of the carbonates it was found that the weight of residue left behind is . Find the mass of in grams in the mixture.
50% studentsanswered this correctly

Important Questions on Some Basic Concepts of Chemistry
MEDIUM
JEE Main/Advance
IMPORTANT
What is the quantity of water (in ) that should be added to methanol to make the mole fraction of methanol as :
MEDIUM
JEE Main/Advance
IMPORTANT
is by mass of solution. If the density is , calculate the molarity.
EASY
JEE Main/Advance
IMPORTANT
A solution containing 0.1 mol of a metal chloride MClx requires 500 ml of 0.8 M AgNO3 solution for complete reaction . Then the value of x is -
MEDIUM
JEE Main/Advance
IMPORTANT
Which is/are correct statements about of
MEDIUM
JEE Main/Advance
IMPORTANT
If of carbon is mixed with of oxygen and is allowed to burn to produce , then:
EASY
JEE Main/Advance
IMPORTANT
The density of air is at . Identify which of the following statement is correct.
MEDIUM
JEE Main/Advance
IMPORTANT
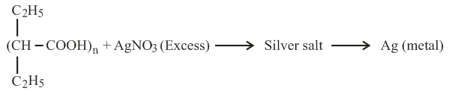
If mole of silver salt is taken and weight of residue obtained is . Then which the following is correct:
MEDIUM
JEE Main/Advance
IMPORTANT
(i)
(ii)
(iii) (a)
(b)
Above steps of reactions occur in a container starting with one mole of and enough water. Find out the limiting reagent in step (i) and calculate maximum moles of gas and that can be produced.
