EASY
Earn 100
A: Kinetic energy of gas molecules is dependent on absolute temperature.
R: (for one mole of a gas).
(a)If both assertion and reasoning are true, and the reason is the correct explanation of the assertion, mark (1).
(b)If both assertion and reasoning are true but the reason is not the correct explanation of the assertion, mark (2).
(c)If the assertion is a true statement and the reason is false, mark (3).
(d)If both assertion and reasoning are false statements, mark (4).
50% studentsanswered this correctly
Important Questions on States of Matter
EASY
MEDIUM
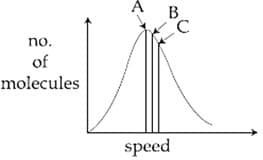
Root mean square speed most proable speed Average speed
EASY
MEDIUM
EASY
MEDIUM
HARD
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
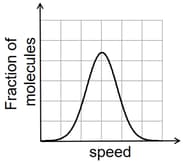
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
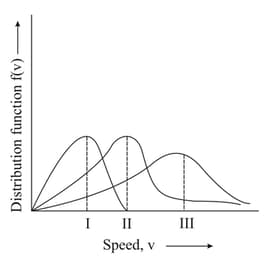
EASY
EASY

