If formation of compound follows the first order of kinetics and after minutes the concentration of was found to be half of its initial concentration. Then the rate constant of the reaction is . The value of is____

Important Questions on Chemical Kinetics
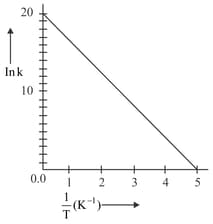
For a reaction, given below is the graph of . The activation energy for the reaction is equal to____.(Given : )
The reaction between and is first order with respect to and zero order with respect to .
| Experiment | |||
| I | |||
| II | |||
| III | |||
| IV |
Examine the data of table and calculate ratio of numerical values of and .
The number of correct statement/s from the following is _____ .
A.Larger the activation energy, smaller is the value of the rate constant.
B. The higher is the activation energy, higher is the value of the temperature coefficient.
C. At lower temperatures, increase in temperature causes more change in the value of than at higher temperature.
D. A plot of vs is a straight line with slope equal to
A student has studied the decomposition of a gas at . He obtained the following data
| Relative |
The order of the reaction is
For the first order reaction the half life is . The time taken for completion of the reaction is mm. (Nearest integer)
Given :
A first order reaction has the rate constant, . The number of correct statement/s from the following is/are Given: .
A. Reaction completes in .
B. The reaction has a half-life of .
C. The time required for completion is 25 times the time required for 90% completion.
D. The degree of dissociation is equal to .
E. The rate and the rate constant have the same unit.
