MEDIUM
JEE Main
IMPORTANT
Earn 100
A molal solution has a degree of dissociation of Its boiling point is equal to that of another solution which contains weight percent of a non electrolytic solute . The molar mass of is _____ u. (Round off to the Nearest Integer). [Density of water
5.88% studentsanswered this correctly

Important Questions on Some Basic Concepts of Chemistry
HARD
JEE Main
IMPORTANT
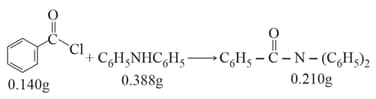
Consider the above reaction. The percentage yield of amide product is (Round off to the Nearest Integer). (Given : Atomic mass :
EASY
JEE Main
IMPORTANT
The reaction of white phosphorus on boiling with alkali in inert atmosphere resulted in the formation of product The reaction of with excess of in aqueous medium gives ________ mole(s) of (Round off to the Nearest Integer).
EASY
JEE Main
IMPORTANT
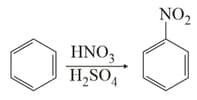
In the above reaction, of benzene on nitration gives of nitrobenzene. The percentage yield of nitrobenzene in the above reaction is _________ . (Round off to the Nearest Integer).
(Given atomic mass : )
MEDIUM
JEE Main
IMPORTANT
When of lead nitrate solution is mixed with of chromic sulphate solution, ____________ moles of lead sulphate precipitate out. (Round off to the Nearest Integer).
HARD
JEE Main
IMPORTANT
Complete combustion of of an organic compound provides of and of . The percentage composition of carbon and hydrogen in organic compound is and ______ respectively. (Round off to the Nearest Integer)
MEDIUM
JEE Main
IMPORTANT
The first and second ionisation enthalpies of a metal are and , respectively. How many moles of and respectively, will be needed to react completely with mole of the metal hydroxide?
MEDIUM
JEE Main
IMPORTANT
of zinc is treated separately with an excess of
dilute hydrochloric acid and
aqueous sodium hydroxide.
The ratio of the volumes of evolved in these two reactions is:
EASY
JEE Main
IMPORTANT
For the following reaction, the mass of water produced from of is:
