EASY
Earn 100
A solution of was titrated with solution. The titration was discontinued after adding of solution. The remaining titration was completed by adding solution. The volume of required for completing the titration is
(a).
(b).
(c).
(d).
50% studentsanswered this correctly
Important Questions on Equilibria
HARD
EASY
EASY
HARD
MEDIUM
Statement I : In the titration between strong acid and weak base methyl orange is suitable as an indicator.
Statement II : For titration of acetic acid with $\mathrm{NaOH}$ phenolphthalein is not a suitable indicator.
In the light of the above statements, choose the most appropriate answer from the options given below:
HARD
HARD
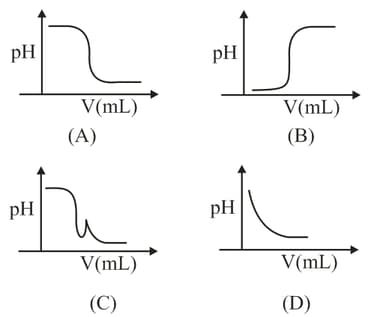
HARD
MEDIUM
MEDIUM
EASY
HARD
(i) What are the initial concentration of and ?
(ii) What is the at the first equivalence point?
(iii) What is the of the mixture before any base is added?
HARD
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
HARD
MEDIUM
MEDIUM
Titration curves for solutions of three weak acids and with ionization constants and respectively are plotted as shown in the figure. Which of the following is/are true?
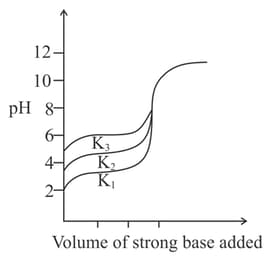
HARD
MEDIUM
EASY
The titration curve for titration of a solution of a diprotic acid with is shown below.
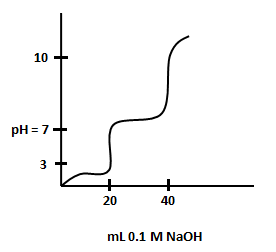
and are approximately

