A ice cube at is placed in a lake whose temperature is . Calculate the change in the entropy of the cube-lake system as the ice cube comes to thermal equilibrium with the lake. The specific heat of ice is . (Hint: Will the ice cube affect the lake temperature?)

Important Questions on Entropy and the Second Law of Thermodynamics
(a) pressure. (b) temperature of the gas. (c) How much work is done by the gas during the expansion?
(d) What is for the expansion? (Hint: Use two simple reversible processes to find )
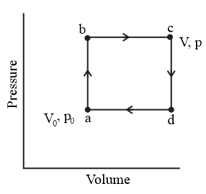
Given figure shows a reversible cycle through which of a monatomic ideal gas is taken. Assume that and . Calculate
(a) the work done during the cycle.
(b) the energy added as heat during stroke and (c) the efficiency of the cycle. (d) What is the efficiency of a Carnot engine operating between the highest and lowest temperatures that occur in the cycle?
(e) Is this greater than or less than the efficiency calculated in (c)?
The efficiency of a particular car engine is when the engine does of work per cycle. Assuming the process is reversible. What are (a) the energy the engine gains per cycle as heat from the fuel combustion and (b) the energy the engine loses per cycle as heat ? If a tune-up increases the efficiency to , what are
(c) and
(d) at the same work value?
(e) What is the net entropy change of the ice original water system as it reaches the equilibrium temperature?
