MEDIUM
Chemistry
IMPORTANT
Earn 100
A orbital has:
(a)Zero angular node and zero radial node
(b)Zero radial node and two angular nodes
(c) radial nodes and angular nodes
(d)Zero radial node and angular nodes
100% studentsanswered this correctly

Important Questions on Structure of Atom
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
Consider following figure and indicating distribution of charge density (electron probability ) with distance
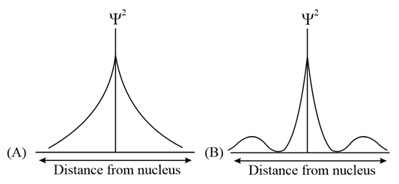
Select the correct statement:
