EASY
Earn 100
A Carnot engine works between constant temperature and of source and sink, respectively. For efficiency to be greatest
(a)both and should be low.
(b)both and should be high.
(c) should be high and should be low.
(d) should be low and should be high.
50% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
EASY
HARD
EASY
(Take cal = Joules)
EASY
EASY
EASY
MEDIUM
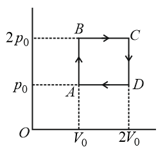
Helium gas goes through a cycle (consisting of two isochoric and two isobaric lines) as shown in figure. The efficiency of this cycle is approximately
MEDIUM
EASY
EASY
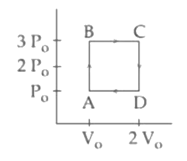
An engine operates by taking a monatomic ideal gas through the cycle shown in the figure. The percentage efficiency of the engine is close to
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
EASY
EASY

