A Carnot reversible engine converts of heat input into work. When the temperature of the sink is reduced by , the efficiency of Carnot's cycle becomes . What is the sum of the temperature (in kelvin) of the source and the sink?

Important Questions on Thermodynamics
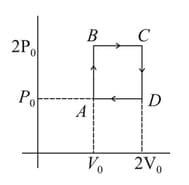
Helium gas goes through a cycle (consisting of two isochoric and isobaric lines) as shown in figure. Efficiency of this cycle is nearly (Assume the gas to be close to ideal gas)
A Carnot engine takes of heat from a reservoir at and gives it to a sink at . The work done by the engine is,
When the absolute temperature of the source of a Carnot heat engine is increased by its efficiency increases by . The new efficiency of the engine is
Consider a reversible engine of efficiency . When the temperature of the sink is reduced by , its efficiency gets doubled. The temperature of the source and sink respectively are
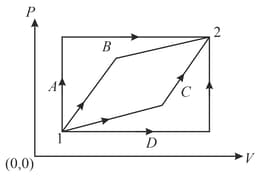
An ideal gas is taken from state to state through optional path and , as shown in the diagram. Let and represent the heat supplied, work done and change in internal energy of the gas, respectively. Then,