A balloon is blown up with of gas has a volume of at . The balloon is filled to of its maximum capacity. Suggest the minimum temperature in Kelvin at which it will burst.

Important Questions on States of Matter
A car tyre has a volume of when inflated. The tyre is inflated to a pressure of at with air. Due to driving the temperature of tyre increases to . What would be the pressure at this temperature?
A car tyre has a volume of when inflated. The tyre is inflated to a pressure of at with air. Due to driving the temperature of tyre increases to . How many litres of air measured at and pressure of should be let out to restore the tyre to at ?
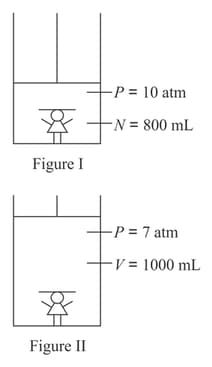
In the diagrams above, a doll is entrapped within a piston and cylinder containing gas. Initial and final conditions are shown in figure and figure , respectively. The volume of the doll is
