EASY
JEE Main/Advance
IMPORTANT
Earn 100
A beam of ultraviolet light of all wavelengths passes through hydrogen gas at room temperature, in the direction. Assume that all photons emitted due to electron transition inside the gas emerge in the direction. Let and denote the lights emerging from the gas in the and directions respectively.
(a)Some of the incident wavelengths will be absent in
(b)Only those wavelengths will be present in which are absent in
(c) will contain some visible light
(d) will contain some infrared light
50% studentsanswered this correctly

Important Questions on Atomic Physics
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
The energy levels of a hypothetical one electron atom are shown in the figure
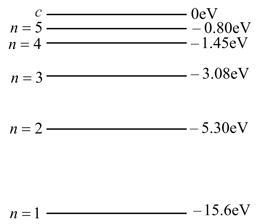
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
