A box contains of liquid water in equilibrium with water vapour at . The equilibrium vapour pressure of water at Torr. When the volume of the box is increased, some of the liquid water evaporates to maintain the equilibrium pressure. If all the liquid water evaporates, then the volume of the box must be ______ litre. [nearest integer]
(Given: _
(Ignore the volume of the liquid water and assume water vapours behave as an ideal gas.)

Important Questions on States of Matter: Gases and Liquids
(Given: The vapour pressure of water at is )
A mixture of hydrogen and helium is contained in a vessel of capacity at bar and . The mass of helium in the mixture is ..... .
(Given : (Atomic masses of and are and , respectively)
'' of molecular oxygen is mixed with of neon . The total pressure of the nonreactive mixture of and in the cylinder is bar. The partial pressure of is bar at the same temperature and volume. The value of '' is
[Given: Molar mass of . Molar mass of ]
The number of statement's, which are correct with respect to the compression of carbon dioxide from point (a) in the Andrews isotherm from the following is _________.
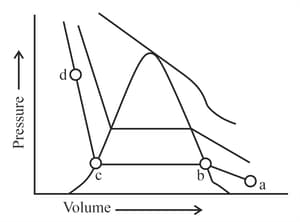
A. Carbon dioxide remains as a gas upto point (b)
B. Liquid carbon dioxide appears at point (c)
C. Liquid and gaseous carbon dioxide coexist between points (b) and (c)
D. As the volume decreases from (b) to (c), the amount of liquid decreases
Based on the given figure, the number of correct statement/s is/are
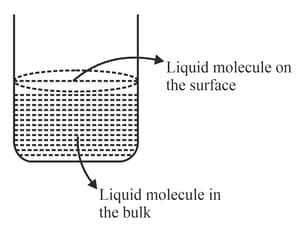
A. Surface tension is the outcome of equal attractive and repulsion forces acting on the liquid molecule in bulk.
B. Surface tension is due to uneven forces acting on the molecules present on the surface.
C. The molecule in the bulk can never come to the liquid surface.
D. The molecules on the surface are responsible for vapour pressure if the system is a closed system.
