EASY
9th Foundation
IMPORTANT
Earn 100
A brief information about two atoms and is given:
Atomic number , Mass number
Atomic number , Mass number
Which of the following is correct about these two atoms?
(a)Electronic configuration of is while that of is .
(b)Both and contain neutrons.
(c) has electron shells while has electron shells.
(d)Both and have valence electrons.
50% studentsanswered this correctly

Important Questions on Structure of Atom
EASY
9th Foundation
IMPORTANT
EASY
9th Foundation
IMPORTANT
EASY
9th Foundation
IMPORTANT
Which of the following are isobars?
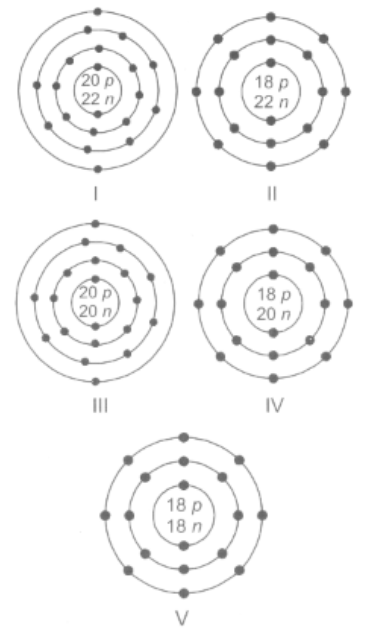
EASY
9th Foundation
IMPORTANT
Statement 1: Bohr's orbits are called stationary orbits.
Statement 2 : Electrons remain stationary in these orbits for some time.
EASY
9th Foundation
IMPORTANT
MEDIUM
9th Foundation
IMPORTANT
Which of the following statements are correct regarding the elements given below?
I The correct order of increasing proton number is
II The correct order of increasing mass number is
III There is difference in the orders of proton number and mass number.
IV The number of protons is equal to number of neutrons in all the given elements.
EASY
9th Foundation
IMPORTANT
MEDIUM
9th Foundation
IMPORTANT
Identify the difference between particles of each given pair.
(I) and
(II) and
(III) and
| I | II | III | |
| (A) | Number of protons | Number of electrons | Number of neutrons |
| (B) | Number of electrons | Number of protons | Number of neutrons |
| (C) | Number of neutrons | Number of electrons | Number of protons |
| (D) | Number of protons | Number of neutrons | Number of electrons |
