A bubble has surface tension . The ideal gas inside the bubble has ratio of specific heats . The bubble is exposed to the atmosphere and it always retains its spherical shape. When the atmospheric pressure is , the radius of the bubble is found to be and the temperature of the enclosed gas is . When the atmospheric pressure is , the radius of the bubble and the temperature of the enclosed gas are and , respectively. Which of the following statement(s) is(are) correct?
Important Questions on Properties of Solid and Liquid
(a) Angle of contact depends upon the inclination of the solid surface to the liquid surface.
(b) If the angle of contact of a liquid and a solid surface is less than then the liquid spreads on the surface of the solid.
(c) Angle of contact increases with increase in temperature of liquid.
(d) The value of angle of contact for pure water and glass is zero.
Given below are two statements: One is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): Clothes containing oil or grease stains cannot be cleaned by water wash.
Reason (R): Because the angle of contact between the oil/grease and water is obtuse. In light of the above statements, choose the correct answer from the option given below.
If two glass plates have water between them and are separated by very small distance (see figure), it is very difficult to pull them apart. It is because the water in between forms cylindrical surface on the side that gives rise to lower pressure in the water in comparison to atmosphere. If the radius of the cylindrical surface is R and surface tension of water is T then the pressure in water between the plates is lower by:
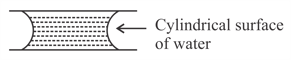
(Take )

