HARD
Earn 100
A bubble of air released by a diver at the bottom of a pool of water becomes larger as it approaches the surface of the water. Assume the temperature is constant and select the true statement. The pressure inside the bubble is
(a)greater near the bottom of the water
(b)greater near the top of the water
(c)same at all depths
(d)cannot be determined from the given data
50% studentsanswered this correctly
Important Questions on States of Matter
HARD
EASY
MEDIUM
MEDIUM
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
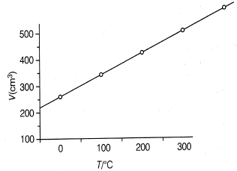
The volume of the gas at is larger than that at by a factor of
EASY
EASY
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
HARD
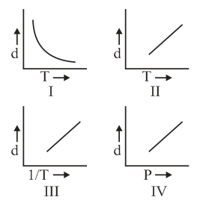
Which one of the following graphs is not correct for ideal gas?
Density, Pressure, Temperature
MEDIUM
HARD
MEDIUM
[Gas constant, ]
MEDIUM
MEDIUM
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?

